The Alpha-Stim AID is a Cranial Electrotherapy Stimulation (CES) device that uses low-level electrical current to safely and effectively treat anxiety, depression, and insomnia. Initially cleared by the FDA in 2024 as a prescriptive, noninvasive treatment, CES has an extensive safety record, with few side effects (less than 1%) and considerable scientific evidence of the significant results patients can achieve.
Body Electric: Electroceuticals and the Future of Medicine Tra...
WATCH: Body Electric: Electroceuticals and the Future of Medicine Trailer This documentary, by independent UK filmmaker Justin Smith, aims to revolutionize the way we think about health and the human body. It features Alpha-Stim technology and includes interviews with Alpha-Stim inventor Dr. Daniel L. Kirsch, EPI President Tracey Kirsch, and VP Science and Education Dr. Jeffrey Marksberry. For more information, and to rent or buy the full film, go to stress.org/bodyelectric/
Posted by Alpha-Stim on Monday, August 28, 2024
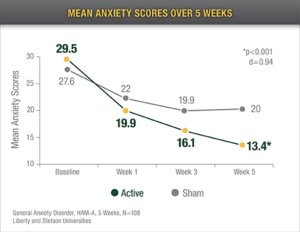
Reduced Anxiety
Eighty-three percent (83%) of the active Alpha-Stim CES group reported a decrease in anxiety of 50% on the HAM-A from baseline to endpoint of study.
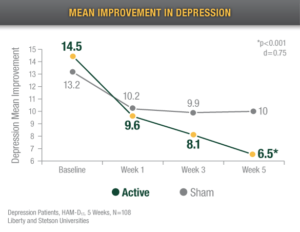
Reduced Depression
Alpha-Stim CES recipients reported an 82% decrease in depression after 5 weeks of treatment.
Reduced Insomnia
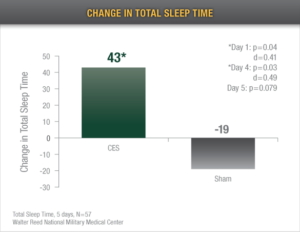
Increased Sleep Time: Alpha-Stim CES recipients demonstrated an average increase of 43 total minutes of sleep time after only 5 treatments.
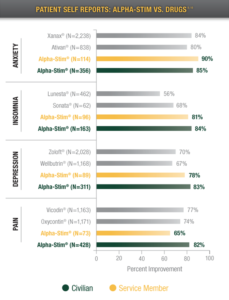
Physician and patient surveys that show 90% of the people who use Alpha-Stim get significant relief. This is supported by over 95 completed independent research studies and published reports, many of which were randomly controlled trials to ensure rigorous testing and clinically validated results. Thats why over 200 Department of Defense (DOD) practitioners and over 92 Veterans Administration (VA) hospitals use Alpha-Stim with military personnel to treat acute, chronic, and post-traumatic pain, anxiety, depression, and insomnia.